Important Science Papers
These selected studies are significant because of their high utility or their analysis which provides perspectives on the policy debate surrounding endocrine issues.
Human-relevant potency threshold (HRPT) for ERα agonism
Borgert, C.J., Matthews, J.C. & Baker, S.P. Arch Toxicol (2018).
The European Commission has recently proposed draft criteria for the identification of endocrine disrupting chemicals (EDCs) that pose a significant hazard to humans or the environment. Identifying and characterizing toxic hazards based on the manner by which adverse effects are produced rather than on the nature of those adverse effects departs from traditional practice and requires a proper interpretation of the evidence regarding the chemical’s ability to produce physiological effect(s) via a specific mode of action (MoA). The ability of any chemical to produce a physiological effect depends on its pharmacokinetics and the potency by which it acts via the various MoAs that can lead to the particular effect. A chemical’s potency for a specific MoA—its mechanistic potency—is determined by two properties: (1) its affinity for the functional components that comprise the MoA, i.e., its specific receptors, enzymes, transporters, transcriptional elements, etc., and (2) its ability to alter the functional state of those components (activity). Using the agonist MoA via estrogen receptor alpha, we illustrate an empirical method for determining a human-relevant potency threshold (HRPT), defined as the minimum level of mechanistic potency necessary for a chemical to be able to act via a particular MoA in humans. One important use for an HRPT is to distinguish between chemicals that may be capable of, versus those likely to be incapable of, producing adverse effects in humans via the specified MoA. The method involves comparing chemicals that have different ERα agonist potencies with the ability of those chemicals to produce ERα-mediated agonist responses in human clinical trials. Based on this approach, we propose an HRPT for ERα agonism of 1E-04 relative to the potency of the endogenous estrogenic hormone 17β-estradiol or the pharmaceutical estrogen, 17α-ethinylestradiol. This approach provides a practical way to address Hazard Identification according to the draft criteria for identification of EDCs recently proposed by the European Commission.CIR Precedents: Endocrine Activity
Cosmetic Ingredient Review (2017).
Concerns have been growing over the past several decades about the potential for exposures to some chemicals to cause adverse health effects by altering the normal functioning of the human endocrine system. The Cosmetic Ingredient Review (CIR) continually monitors developments of the research and the regulation of such substances as a matter of long-standing policy. CIR safety assessment reports include data from in vitro (e.g., estrogen-receptor binding) and in vivo (e.g., uterotrophic) assays that address the potential for ingredients to bind to and interact with endocrine receptors and other components of the endocrine system, as well as reproductive toxicity studies that identify adverse responses for safety assessment. The CIR Expert Panel considers ingredients that have demonstrated endocrine activity in such tests as potential endocrine disrupting chemicals (EDCs), depending on the relevance, quality and concordance of the available studies, the doses and concentrations tested and the dose- or concentration-response relationships observed in such studies, the affinities of the ingredients for endocrine receptors or other components of the endocrine system, the potency of endocrine-active ingredients compared with endogenous hormones, and other important factors that contribute to an assessment of the overall weight-of-the-evidence (WoE). Such assessments depend, at the outset, on a clear definition of what constitutes an EDC, understanding of the distinction between endocrine activity and endocrine disruption, and differentiation of endocrine-mediated effects from other likely mechanisms of action (MOAs).Human cost burden of exposure to endocrine disrupting chemicals. A critical review
Bond, G.G. & Dietrich, D.R. Arch Toxicol (2017). doi:10.1007/s00204-017-1985-y
Recently published papers have alleged that exposures to endocrine disrupting chemicals (EDCs) are causing substantial disease burdens in the EU and US and are consequently costing society hundreds of billions of dollars annually. To date, these cost estimates have not undergone adequate scientific scrutiny, but nevertheless are being used aggressively in advocacy campaigns in an attempt to fundamentally change how chemicals are tested, evaluated and regulated. Consequently, we critically evaluated the underlying methodology and assumptions employed by the chief architects of the disease burden cost estimates. Since the vast majority of their assigned disease burden costs are driven by the assumption that “loss of IQ” and “increased prevalence of intellectual disability” are caused by exposures to organophosphate pesticides (OPPs) and brominated flame retardants (PBDEs), we have taken special care in describing and evaluating the underlying toxicology and epidemiology evidence that was relied upon. Unfortunately, our review uncovered substantial flaws in the approach taken and the conclusions that were drawn. Indeed, the authors of these papers assumed causal relationships between putative exposures to EDCs and selected diseases, i.e., “loss of IQ” and “increased prevalence of intellectual disability”, despite not having established them via a thorough evaluation of the strengths and weaknesses of the underlying animal toxicology and human epidemiology evidence. Consequently, the assigned disease burden costs are highly speculative and should not be considered in the weight of evidence approach underlying any serious policy discussions serving to protect the public and regulate chemicals considered as EDCs.Evaluating the credibility of histopathology data in environmental endocrine toxicity studies
Environ Toxicol Chem 2017;36:601–611
Agencies responsible for environmental protection are tasked with developing regulatory guidance that is based on the best available scientific evidence. Histopathology is a common endpoint in toxicologic bioassays; however, because of the subjective nature of this endpoint, and the advanced level of specialized training required for its effective utilization, the reliability of histopathology data can be inconsistent. Consequently, mechanisms for evaluating such data on a case-by-case basis are needed. The purposes of the present review are to describe a methodology that can be used to evaluate the credibility of histopathology findings and to discuss the results of such assessments as applied to real-world data collected from the scientific literature. A key outcome of these efforts was the finding that only 54% of the studies examined contained histopathology data that were considered to be either highly credible or credible, whereas data in 46% of those studies were of equivocal, dubious, or no credibility. In addition, the results indicated that the quality of the data examined tended to decline during the past 15 yr. The ultimate goals of the present review are to draw attention to reliability issues that can affect histopathology results, provide recommendations to improve the quality of this endpoint, and suggest an approach for the expeditious and judicious use of histopathology data in the weight-of-evidence determinations required for hazard and/or risk assessment. This exercise was conducted initially as part of a SETAC Pellston Workshop™ entitled “Environmental Hazard and Risk Assessment Approaches for Endocrine-Active Chemicals (EHRA): Developing Technical Guidance Based on Case Studies to Support Decision Making” that was held in Pensacola, Florida (USA) from 31 January to 5 February 2016.Uncertainties in biological responses that influence hazard and risk approaches to the regulation of endocrine active substances
Integr Environ Assess Manag 2017;13:293–301.
Endocrine-disrupting substances (EDS) may have certain biological effects including delayed effects, multigenerational effects, and may display nonmonotonic dose–response (NMDR) relationships that require careful consideration when determining environmental hazards. Endocrine disrupting substances can have specific and profound effects when exposure occurs during sensitive windows of the life cycle (development, reproduction). This creates the potential for delayed effects that manifest when exposure has ceased, possibly in a different life stage. This potential underscores the need for testing in appropriate (sensitive) life stages and full life cycle designs. Such tests are available in the Organisation for Economic Co-operation and Development (OECD) tool box and should be used to derive endpoints that can be considered protective of all life stages. Similarly, the potential for effects to be manifest in subsequent generations (multigenerational effects) has also been raised as a potential issue in the derivation of appropriate endpoints for EDS. However, multigenerational studies showing increasing sensitivity of successive generations are uncommon. Indeed this is reflected in the design of new higher tier tests to assess endocrine active substances (EAS) that move to extended one-generation designs and away from multi-generational studies. The occurrence of NMDRs is also considered a limiting factor for reliable risk assessment of EDS. Evidence to date indicates NMDRs are more prevalent in in vitro and mechanistic data, not often translating to adverse apical endpoints that would be used in risk assessment. A series of steps to evaluate NMDRs in the context of endocrine hazard and risk assessment procedures is presented. If careful consideration of delayed, multigenerational effects and NMDRs is made, it is feasible to assess environmental endocrine hazards and derive robust apical endpoints for risk assessment procedures ensuring a high level of environmental protection. Integr Environ Assess Manag 2017;13:293–301.Population-relevant endpoints in the evaluation of endocrine-active substances (EAS) for ecotoxicological hazard and risk assessment
Integr Environ Assess Manag 2017;13:317–330
For ecotoxicological risk assessment, endocrine disruptors require the establishment of an endocrine mode of action (MoA) with a plausible link to a population-relevant adverse effect. Current ecotoxicity test methods incorporate mostly apical endpoints although some also include mechanistic endpoints, subcellular-through-organ level, which can help establish an endocrine MoA. However, the link between these endpoints and adverse population-level effects is often unclear. The case studies of endocrine-active substances (EAS) (tributyltin, ethinylestradiol, perchlorate, trenbolone, propiconazole, and vinclozolin) evaluated from the Society of Environmental Toxicology and Chemistry (SETAC) Pellston Workshop® “Ecotoxicological Hazard and Risk Assessment Approaches for Endocrine-Active Substances (EHRA)” were used to evaluate the population relevance of toxicity endpoints in various taxa according to regulatory endocrine-disruptor frameworks such as the Organisation for Economic Co-operation and Development (OECD) Conceptual Framework for Testing and Assessment of Endocrine Disruptors. A wide variety of potentially endocrine-relevant endpoints were identified for mollusks, fish, amphibians, birds, and mammals, although the strength of the relationship between test endpoints and population-level effects was often uncertain. Furthermore, testing alone is insufficient for assessing potential adaptation and recovery processes in exposed populations. For this purpose, models that link effects observed in laboratory tests to the dynamics of wildlife populations appear to be necessary, and their development requires reliable and robust data. As our understanding of endocrine perturbations and key event relationships improves, adverse population-level effects will be more easily and accurately predicted. Integr Environ Assess Manag 2017;13:317–330Challenges in assigning endocrine-specific modes of action: Recommendations for researchers and regulators
Integr Environ Assess Manag 2017;13:280–292
As regulatory programs evaluate substances for their endocrine-disrupting properties, careful study design and data interpretation are needed to distinguish between responses that are truly endocrine specific and those that are not. This is particularly important in regulatory environments where criteria are under development to identify endocrine-disrupting properties to enable hazard-based regulation. Irrespective of these processes, most jurisdictions use the World Health Organization/International Programme on Chemical Safety definition of an endocrine disruptor, requiring that a substance is demonstrated to cause a change in endocrine function that consequently leads to an adverse effect in an intact organism. Such a definition is broad, and at its most cautious might capture many general mechanisms that would not specifically denote an endocrine disruptor. In addition, endocrine responses may be adaptive in nature, designed to maintain homeostasis rather than induce an irreversible adverse effect. The likelihood of indirect effects is increased in (eco)toxicological studies that require the use of maximum tolerated concentrations or doses, which must produce some adverse effect. The misidentification of indirect effects as truly endocrine mediated has serious consequences for prompting animal- and resource-intensive testing and regulatory consequences. To minimize the risk for misidentification, an objective and transparent weight-of-evidence procedure based on biological plausibility, essentiality, and empirical evidence of key events in an adverse outcome pathway is recommended to describe the modes of action that may be involved in toxic responses in nontarget organisms. Confounding factors such as systemic toxicity, general stress, and infection can add complexity to such an evaluation and should be considered in the weight of evidence. A recommended set of questions is proffered to help guide researchers and regulators in discerning endocrine and nonendocrine responses. Although many examples provided in this study are based on ecotoxicology, the majority of the concepts and processes are applicable to both environmental and human health assessments. Integr Environ Assess Manag 2017;13:280–292.Current limitations and recommendations to improve testing for the environmental assessment of endocrine active substances
Integr Environ Assess Manag 2017;13:302–316
In the present study, existing regulatory frameworks and test systems for assessing potential endocrine active chemicals are described, and associated challenges are discussed, along with proposed approaches to address these challenges. Regulatory frameworks vary somewhat across geographies, but all basically evaluate whether a chemical possesses endocrine activity and whether this activity can result in adverse outcomes either to humans or to the environment. Current test systems include in silico, in vitro, and in vivo techniques focused on detecting potential endocrine activity, and in vivo tests that collect apical data to detect possible adverse effects. These test systems are currently designed to robustly assess endocrine activity and/or adverse effects in the estrogen, androgen, and thyroid hormone signaling pathways; however, there are some limitations of current test systems for evaluating endocrine hazard and risk. These limitations include a lack of certainty regarding: 1) adequately sensitive species and life stages; 2) mechanistic endpoints that are diagnostic for endocrine pathways of concern; and 3) the linkage between mechanistic responses and apical, adverse outcomes. Furthermore, some existing test methods are resource intensive with regard to time, cost, and use of animals. However, based on recent experiences, there are opportunities to improve approaches to and guidance for existing test methods and to reduce uncertainty. For example, in vitro high-throughput screening could be used to prioritize chemicals for testing and provide insights as to the most appropriate assays for characterizing hazard and risk. Other recommendations include adding endpoints for elucidating connections between mechanistic effects and adverse outcomes, identifying potentially sensitive taxa for which test methods currently do not exist, and addressing key endocrine pathways of possible concern in addition to those associated with estrogen, androgen, and thyroid signaling. Integr Environ Assess Manag 2017;13:302–316.Ecotoxicological hazard and risk assessment of endocrine active substances
Integr Environ Assess Manag 2017;13:264–266.
This collection of papers provides state-of-the-art science on a complex topic that has been challenging for scientists and regulators for a long time. The papers emanated from the Society of Environmental Toxicology and Chemistry (SETAC) Pellston Workshop® Environmental Hazard and Risk Assessment Approaches for Endocrine-Active Substances (EHRA). Forty-eight international experts met in early February 2016 to discuss whether the environmental risks posed by endocrine-disrupting substances (EDS) can be reliably assessed. The primary conclusion of the workshop was that if data on environmental exposure, effects on sensitive species and life-stages, delayed effects, and effects at low concentrations are robust, initiating environmental risk assessment of EDS is scientifically sound and reliable. Integr Environ Assess Manag 2017;13:264–266.Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances
Integr Environ Assess Manag 2017;13:267–279.
A SETAC Pellston Workshop® “Environmental Hazard and Risk Assessment Approaches for Endocrine-Active Substances (EHRA)” was held in February 2016 in Pensacola, Florida, USA. The primary objective of the workshop was to provide advice, based on current scientific understanding, to regulators and policy makers; the aim being to make considered, informed decisions on whether to select an ecotoxicological hazard- or a risk-based approach for regulating a given endocrine-disrupting substance (EDS) under review. The workshop additionally considered recent developments in the identification of EDS. Case studies were undertaken on 6 endocrine-active substances (EAS—not necessarily proven EDS, but substances known to interact directly with the endocrine system) that are representative of a range of perturbations of the endocrine system and considered to be data rich in relevant information at multiple biological levels of organization for 1 or more ecologically relevant taxa. The substances selected were 17α-ethinylestradiol, perchlorate, propiconazole, 17β-trenbolone, tributyltin, and vinclozolin. The 6 case studies were not comprehensive safety evaluations but provided foundations for clarifying key issues and procedures that should be considered when assessing the ecotoxicological hazards and risks of EAS and EDS. The workshop also highlighted areas of scientific uncertainty, and made specific recommendations for research and methods-development to resolve some of the identified issues. The present paper provides broad guidance for scientists in regulatory authorities, industry, and academia on issues likely to arise during the ecotoxicological hazard and risk assessment of EAS and EDS. The primary conclusion of this paper, and of the SETAC Pellston Workshop on which it is based, is that if data on environmental exposure, effects on sensitive species and life-stages, delayed effects, and effects at low concentrations are robust, initiating environmental risk assessment of EDS is scientifically sound and sufficiently reliable and protective of the environment. In the absence of such data, assessment on the basis of hazard is scientifically justified until such time as relevant new information is available. Integr Environ Assess Manag 2017;13:267–279.Upholding science in health, safety and environmental risk assessments and regulations
Toxicology
Volume 371, 14 September 2016, Pages 12–16
http://doi.org/10.1016/j.tox.2016.09.005
A public appeal has been advanced by a large group of scientists, concerned that science has been misused in attempting to quantify and regulate unmeasurable hazards and risks.1 The appeal recalls that science is unable to evaluate hazards that cannot be measured, and that science in such cases should not be invoked to justify risk assessments in health, safety and environmental regulations.
The appeal also notes that most national and international statutes delineating the discretion of regulators are ambiguous about what rules of evidence ought to apply. Those statutes should be revised to ensure that the evidence for regulatory action is grounded on the standards of the scientific method, whenever feasible. When independent scientific evidence is not possible, policies and regulations should be informed by publicly debated trade-offs between socially desirable uses and social perceptions of affordable precaution. This article explores the premises, implications and actions supporting the appeal and its objectives.
The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis
Personal care products and endocrine disruption: A critical review of the literature
Witorsch RJ. Crit Rev Toxicol. 2010 Nov;40 Suppl 3:1-30. doi: 10.3109/10408444.2010.515563
This article reviews laboratory and epidemiological research into the endocrine disruptive effects of components of personal care products, namely, phthalate esters, parabens, ultraviolet (UV) filters, polycyclic musks, and antimicrobials. High doses of phthalates in utero can produce “phthalate syndrome,” demasculinizing effects in male rat offspring due to impaired testosterone production by fetal testes. However, evidence linking phthalate exposure to similar effects in humans appears inconclusive. Furthermore, phthalate exposure derived from personal care products is within safe limits and its principal bioavailable phthalate, diethyl phthalate (DEP), does not produce “phthalate syndrome.” Parabens exhibit very weak estrogen activity in vitro and in vivo, but evidence of paraben-induced developmental and reproductive toxicity in vivo lacks consistency and physiological coherence. Evidence attempting to link paraben exposure with human breast cancer is nonexistent. Select UV filters at high doses produce estrogenic, antithyroid, and other effects in rats in vivo. Again, no evidence links UV filter exposure to endocrine disruptive effects in humans. Some polycyclic musks weakly bind to estrogen, androgen, or progestin receptors and exhibit primarily antagonistic activity in vitro, which for the most part, has yet to be confirmed in vivo in mammals. The antimicrobials triclocarban and triclosan evoke weak responses mediated by aryl hydrocarbon, estrogen, and androgen receptors in vitro, which require confirmation in vivo. Preliminary observations suggest a novel interaction between triclocarban and testosterone. In conclusion, although select constituents exhibit interactions with the endocrine system in the laboratory, the evidence linking personal care products to endocrine disruptive effects in humans is for the most part lacking.Effects of elevated glucocorticoids on reproduction and development: relevance to endocrine disruptor screening.
Witorsch RJ. Crit Rev Toxicol. 2016;46(5):420-36. doi: 10.3109/10408444.2016.1140718. Epub 2016 Feb 25.
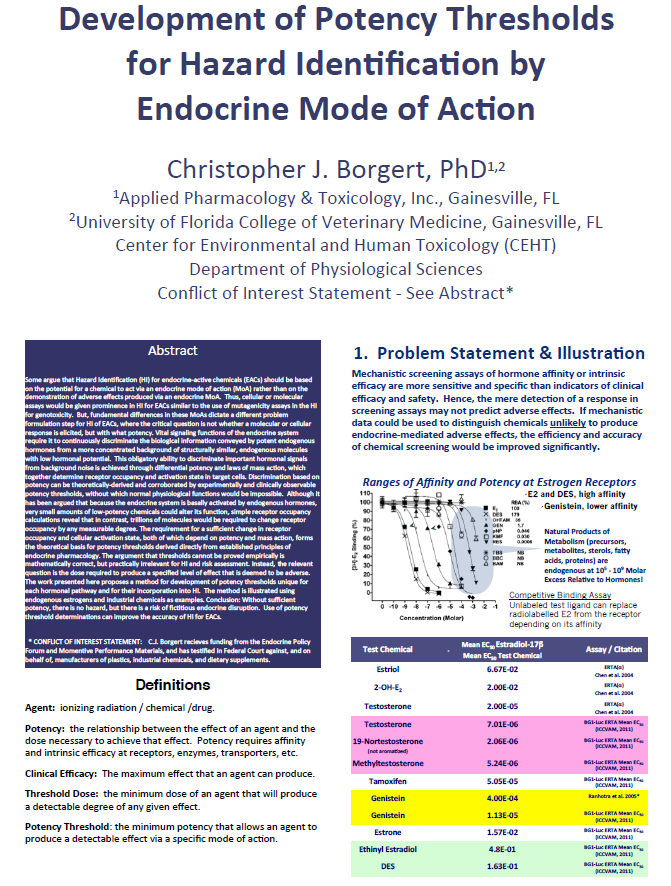
This article reviews the influence of the hypothalamo-pituitary-adrenocortical (HPA) axis on mammalian male and female reproduction and development of offspring and its potential impact on the identification of endocrine disruptive chemicals by in vivo assays. In the adult male rat and baboon, stress suppresses testosterone secretion via a direct inhibitory effect of elevated glucocorticoids on Leydig cells. In adult female sheep, stress disrupts reproductive function via multi-stage mechanisms involving glucocorticoid-mediated suppression of LH secretion, LH action on the ovary and the action of estradiol on its target cells (e.g., uterus). While physiological concentrations of endogenous glucocorticoids are supportive of fetal development, excessive glucocorticoids in utero (i.e., maternal stress) adversely affect mammalian offspring by "programing" abnormalities that are primarily manifest postpartum. The influence of stress on reproduction and development can also be mediated by 11β-hydroxysteroid dehydrogenase (HSD), a bi-directional oxidative:reductive pathway, which governs the balance between biologically active (reduced) endogenous glucocorticoid and inactive (oxidized) metabolites. This pathway is mediated primarily by two isozymes, 11β - HSD1 (reductase) and 11β-HSD2 (oxidase) which act both in an intracrine (intracellular) and endocrine (systemic) fashion. The 11β-HSD pathway appears to play a variety of physiological roles in mammalian reproduction and development and is a target for selected xenobiotics. The effects of the HPA axis on mammalian reproduction and development are potential confounders for in vivo bioassays in rodents employed to identify endocrine disruptive chemicals. Accordingly, consideration of the impact of the HPA axis should be incorporated into the design of bioassays for evaluating endocrine disruptors.Development of Potency Thresholds for Hazard Identification by Endocrine Mode of Action
Christopher J. Borgert, PhD
Some argue that Hazard Identification (HI) for endocrine-active chemicals (EACs) should be based on the potential for a chemical to act via an endocrine mode of action (MoA) rather than on the demonstration of adverse effects produced via an endocrine MoA. Thus, cellular or molecular assays would be given prominence in HI for EACs similar to the use of mutagenicity assays in the HI for genotoxicity. But, fundamental differences in these MoAs dictate a different problem formulation step for HI of EACs, where the critical question is not whether a molecular or cellular response is elicited, but with what potency. Vital signaling functions of the endocrine system require it to continuously discriminate the biological information conveyed by potent endogenous hormones from a more concentrated background of structurally similar, endogenous molecules with low hormonal potential. This obligatory ability to discriminate important hormonal signals from background noise is achieved through differential potency and laws of mass action, which together determine receptor occupancy and activation state in target cells. Discrimination based on potency can be theoretically-derived and corroborated by experimentally and clinically observable potency thresholds, without which normal physiological functions would be impossible. Although it has been argued that because the endocrine system is basally activated by endogenous hormones, very small amounts of low-potency chemicals could alter its function, simple receptor occupancy calculations reveal that in contrast, trillions of molecules would be required to change receptor occupancy by any measurable degree. The requirement for a sufficient change in receptor occupancy and cellular activation state, both of which depend on potency and mass action, forms the theoretical basis for potency thresholds derived directly from established principles of endocrine pharmacology. The argument that thresholds cannot be proved empirically is mathematically correct, but practically irrelevant for HI and risk assessment. Instead, the relevant question is the dose required to produce a specified level of effect that is deemed to be adverse. The work presented here proposes a method for development of potency thresholds unique for each hormonal pathway and for their incorporation into HI. The method is illustrated using endogenous estrogens, anti-androgens, and industrial chemicals as examples. Conclusion: Without sufficient potency, there is no hazard, but there is a risk of fictitious endocrine disruption. Use of potency threshold determinations can improve the accuracy of HI for EACs.Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High Throughput Screening Assays for the Estrogen Receptor.
Judson RS et al. Toxicol Sci. 2015 Aug 13. pii: kfv168. [Epub ahead of print]
Further documentation of the ToxCast ER AUC model is detailed in this report. Details include documentation of how the model performs in predicting ER agonist or antagonist activity, as well as approaches to identify false positive responses caused by assay interference. In applying this method to 1,812 commercial and environmental chemicals, 111 (6.1%) were predicted to be ER active in agonist or antagonist mode. The authors discuss how this model can be used to distinguish ER active chemicals with environmental chemicals with human exposure potential from non-ER active substances. Substances with negligible activity would be deprioritized, while prioritized substances would be candidates for additional in vivo endocrine screening.Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model.
Browne P et al. Environ Sci Technol. 2015 Jul 21;49 (14):8804-14. doi: 10.1021/acs.est.5b02641.
The authors report on the performance of a model developed using HTS1 assays of estrogenic activity to predict the uterotrophic response in vivo in rodents. This computational model, the ToxCast ER AUC model, integrates results from 18 separate assays to derive bioactivity scores. The model was shown to be 86% to 93% accurate in predicting reference chemicals. In addition, the model predicted results of the EDSP Tier 1 guideline and other uterotrophic studies with 84% to 100% accuracy. This performance is sufficient for EPA to accept the ToxCast ER model data for chemicals as alternatives for EDSP Tier 1 ER binding, ER transactivation, and uterotrophic assays.An Exposure:Activity Profiling Method for Interpreting High-throughput Screening Data for Estrogenic Activity – – Proof of Concept.
Becker RA et al. Regul Toxicol Pharmacol. 2015 Apr; 71(3):398-408 doi: 10.1016/j.yrtph.2015.01.008.
This article illustrates how to integrate information on exposure with results of rapid high-throughput in vitro screening assays for estrogenic activity. This exposure:activity profiling is accomplished by calculating the exposure:activity ratios (EARs) using human exposure estimates and AC50 values for a range of chemicals tested in a suite of seven estrogenic assays in ToxCast™ and Tox21. Additional context is provided by comparing chemical-specific EARs to the EAR of the ubiquitous dietary phytoestrogen, genistein (GEN) to calculate relative estrogenic exposure:activity quotients (REEAQ) . For risk-based prioritization, substances with small EARs and REEAQs would indicate low priority for further endocrine screening or testing.Developing Scientific Confidence in HTS-derived Prediction Models: Lessons Learned from an Endocrine Case Study.
Cox et al. Regul Toxicol Pharmacol. 2014 Aug; 69(3):443-50. doi: 0.1016/j.yrtph.2014.05.010.
Scientific confidence in high-throughput screening (HTS) methods needs to be established prior to regulatory use. This investigation focused on a case study of classification models originally developed by the EPA that used HTS results to predict in vivo endocrine endpoints. EPA’s classification models were independently recapitulated, and then extended to more robust cross validation models. Cross validation models (based on a set of endocrine ToxCast™ assays and guideline in vivo endocrine screening studies) were shown to have balanced accuracies from 79% to 85% for androgen and estrogen, but only 23% to 50% for thyroid and steroidogenesis. These results indicate the estrogen and androgen models are quite promising for initial use in setting priorities for endocrine screening. Based on the lessons learned, the authors proposed a framework for organizing and documenting scientific confidence in HTS assays and the prediction models derived therefrom.The Screening of Everyday Life Chemicals in Validated Assays Targeting the Pituitary-gonadal Axis.
H. Tinwell, et al., Regul. Toxicol. Pharmacol. (2013) 66(2): 184–196.
The authors tested ten structurally diverse chemicals (vitamins C, B9, B6, B3, sucrose, caffeine, gingerol, xanthan gum, paracetamol, and ibuprofen) deemed intrinsic to modern life but not considered as endocrine active, in several commonly used screening assays designed to identify industrial chemicals as endocrine active. They found that most of the chemicals tested as endocrine active in at least one of the screening tests. They concluded that to avoid regulation of an overwhelming number of chemicals, a WoE approach, combining hazard identification and characterization with exposure considerations, is needed to identify those chemicals of real regulatory concern.Trends in Global Semen Parameter Values.
H. Fisch and S.R. Braun, Asian J. Androl. (2013) 15: 169–173;
The authors conclude that allegations of a worldwide decline in semen parameter values have not withstood scientific scrutiny. Reported declines in semen parameter values are likely to be either highly local phenomena with an unknown etiology or the result of methodological errors arising from attempts to observe highly variable physical attributes (semen characteristics) with relatively low-resolution tools (retrospective analysis of nonrandomized study populations). The authors summarize methodological flaws in an influential 1992 paper and review studies that have been published since 1992. Of the 35 major studies of time trends in semen quality reviewed, eight (a total of 18,109 men) suggest a decline in semen quality; 21 (112,386 men) show either no change or an increase in semen quality; and six (26,007 men) show ambiguous or conflicting results. The cause (or causes) of the geographical and temporal variations in semen parameter values reported by these diverse studies deserve further investigation. The authors conclude that the data supporting a role for ‘endocrine disruptors’ in the alleged ‘decline’ in semen parameters is weak.Toxicity Evaluation of Bisphenol A Administered by Gavage to Sprague-Dawley Rats From Gestation Day 6 Through Postnatal Day 90.
K.B. Delclos, et al., Toxicol. Sci. (2014) 139(1): 174-197.
This study was authored by scientists from the U.S. National Center for Toxicological Research which is a research arm of the FDA. The primary goals of the subchronic study were to identify adverse effects induced by orally (gavage) administered BPA at low doses (well below the no observed adverse effect level, or NOAEL), to characterize the dose response for such effects and to determine doses for a subsequent chronic study. Sprague-Dawley rat mothers were dosed daily from gestation day 6 until the start of labor, and their pups were directly dosed from day 1 after birth to termination. The primary focus was on seven equally spaced BPA doses (2.5–2700 μg/kg bw/day). Also included were a naïve control, two doses of ethinyl estradiol (EE2), to demonstrate the estrogen responsiveness of the animal model, and two high BPA doses (100,000 and 300,000 μg/kg bw/day) expected from guideline studies to produce adverse effects. Clear adverse effects of BPA, including depressed gestational and postnatal body weight gain, effects on the ovary (increased cystic follicles, depleted corpora lutea, and antral follicles), and serum hormones (increased serum estradiol and prolactin and decreased progesterone), were observed only at the two highest doses of BPA. BPA-induced effects partially overlapped those induced by EE2, consistent with the known weak estrogenic activity of BPA.Two-generation Reproductive Toxicity Study of Dietary Bisphenol A in CD-1 (Swiss) Mice.
R.W. Tyl, et al., Toxicol. Sci. (2008) 104(2) : 362–384.
A two-generation study of dietary exposure to BPA was conducted in mice at six different dose levels ranging from very low to high to test whether BPA could cause reproductive or developmental effects and the low dose hypothesis (i.e., that lower exposures to endocrine active substances are more harmful than higher exposures). There were no BPA-related effects observed on an extensive range of reproductive and developmental outcomes including: adult mating, fertility or gestational indices, ovarian primordial follicle counts, estrous cyclicity, precoital interval, offspring sex ratios or postnatal survival, sperm parameters or reproductive organ weights or histopathology (including the testes and prostate). At lower doses there were no treatment-related effects and no evidence to support a low dose hypothesis (e.g., no NMDR curves for any parameter). The authors concluded that BPA is not considered a selective reproductive or developmental toxicant in mice.Risk Assessment of Endocrine Active Chemicals: Identifying Chemicals of Regulatory Concern.
R. Bars, et al., Regul. Toxicol. Pharmacol. (2012) 64(1): 143–154.
A European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) task force was formed to propose scientific criteria on how to identify and evaluate endocrine activity and disruption within EU regulations on pesticides, biocides and industrial chemicals. The resulting ECETOC technical report and an associated workshop with participation by academic, regulatory and private sector scientists presented a science-based concept on how to identify endocrine activity and disrupting properties of chemicals for both human health and the environment. Specific scientific criteria for the determination of endocrine activity and disrupting properties that integrate information from both regulatory (eco) toxicity studies and mechanistic/screening studies were proposed. These criteria combined the nature of the adverse effects detected in studies which give concern for endocrine toxicity with an understanding of the mode of action of toxicity so that adverse effects can be explained scientifically. A key element in the data evaluation is the consideration of all available information in a WoE approach. However, to be able to discriminate chemicals with endocrine properties of low concern from those of higher concern (for regulatory purposes) the task force recognized that the concept needed further refinement. Following a discussion of the key factors at a second workshop, the task force developed further guidance, which is presented in this paper. For human health assessments these factors include the relevance to humans of the endocrine mechanism of toxicity, the specificity of the endocrine effects with respect to other potential toxic effects, the potency of the chemical to induce endocrine toxicity and consideration of exposure levels. For ecotoxicological assessments the key considerations include specificity and potency, but also extend to the consideration of population relevance and negligible exposure.Is It Time to End Concerns over the Estrogenic Effects of Bisphenol A?
R.M. Sharpe, Toxicol. Sci. (2010) 114(1): 1-4.
Richard Sharpe of the U.K. Medical Research Council offers a commentary in the wake of another study (Ryan et al., 2009) that investigated and failed to find low-dose effects of BPA. Sharpe discusses the central importance of the repeatability of experiments and how we should react when a study proves to be unrepeatable. For more than a decade, scientific controversy swirled over some initial reports of low-dose effects of BPA and whether or not BPA exerts adverse estrogenic effects in animal studies. Critics of those so called “low dose” studies claimed that the studies utilized an insufficient number of dose groups to establish a clear dose-response relationship, used small numbers of animals, and/or failed to appropriately account for possible litter effects in the analysis of the data. More recent studies included the one by Ryan et al. addressed these shortcomings in their design and execution and yet failed to find low-dose effects. Sharpe concludes, "Fundamental, repetitive work on bisphenol A has sucked in tens, probably hundreds, of millions of dollars from government bodies and industry which, at a time when research money is thin on the ground, looks increasingly like an investment with a nil return. All it has done is to show that there is a huge price to pay when initial studies are adhered to as being correct when the second phase of scientific peer review, namely, the inability of other laboratories to repeat the initial studies, says otherwise.”In utero and Lactational Exposure to Bisphenol A, In Contrast to Ethinyl Estradiol, Does Not Alter Sexually Dimorphic Behavior, Puberty, Fertility, and Anatomy of Female LE Rats.
B.C. Ryan, et al., Toxicol. Sci. (2010) 114(1): 133-48.
This study, conducted by scientists from the EPA, was designed to determine if maternal exposure to relatively low oral doses of oral contraceptive ethinyl estradiol (EE2) or bisphenol A (BPA) in utero and during lactation would alter sexual behaviors or alter the age of puberty or reproductive function in female rat offspring. It was intended to test whether BPA could cause such effects and particularly whether BPA and the oral contraceptive pill could cause more harm at low doses to test the low dose hypothesis. Pregnant rats were exposed to a wide range of doses of EE2 (0.05-50 microg/kg/day), or BPA (2, 20, and 200 microg/kg/day) from day 7 of gestation to postnatal day (PND) 18, and the female offspring were studied. EE2 (50 microg/kg/day) caused a number of adverse effects both in structure and function, but only at doses in the range of those that were pharmacology relevant. By contrast, BPA caused no adverse effects.Low-dose Effects and Non-monotonic Dose-responses of Endocrine Disrupting Chemicals: Has the Case Been Made?
L.R. Rhomberg, J.E. Goodman, Regul. Toxicol. Pharmacol. (2012) 64(1): 130–133.
The authors critique a paper by Vandenberg et al. (2012) which claimed that “most if not all [endocrine-disrupting chemicals (EDCs)] are likely to have low-dose effects” and “non-monotonicity is a common occurrence after exposures to hormones and EDCs in cell culture and animals and across human populations.” The authors note, for example, Vandenberg et al. present examples as anecdotes without attempting to review all available pertinent data, selectively citing studies without evaluating most of them or examining whether their putative examples are consistent and coherent with other relevant information. They assume that any statistically significant association indicates causation of an adverse effect, and their limited evaluation of specific studies is not done uniformly (i.e., studies with positive results are evaluated differently than those with null results). They also do not evaluate whether exposures in studies are truly “low-dose” and relevant to humans. They propose a number of different NMDR curves, but do not consider reasons for why they should be expected to apply generally across species. Overall, the authors conclude that Vandenberg et al. put forth a number of asserted illustrations of their two conclusions without providing sufficient evidence to make the case for either, and while overlooking evidence that suggests the contrary.State of the Science Evaluation: Non-monotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures.
U.S. Environmental Protection Agency, June 2013
Given allegations that EDCs may not display traditional dose-response curves (i.e., they may behave differently at lower doses) and the consequent implication that current approaches for regulating chemicals may not be adequate for EDCs, EPA chartered a team of U.S. Food and Drug Administration (FDA) and EPA scientists to develop a state of the science evaluation of the degree to which NMDRs are evidenced in the scientific literature and the extent to which they may impact EPA’s chemical testing and risk assessment policies and procedures. They found that NMDRs after exposure to synthetic chemicals do occur in biological systems, but are generally not common. Where NMDRs were observed, they were more likely to occur at the molecular level rather than at the level of the subsequent adverse outcomes. Current testing approaches successfully identify the potential for hazards due to exposure to the chemical of concern and, based on the current evaluation, are highly unlikely to mischaracterize a chemical that has the potential to adversely affect the endocrine system due to an NMDR. EPA sought and received comments on their technical report from an external advisory group and is in the process of addressing comments prior to issuing a final report.Information Quality in Regulatory Decision-making: Peer Review versus Good Laboratory Practice.
L.S. McCarty, et al., Environ. Health Perspect. (2012) 120(7): 927-934.
This article presents a review of Good Laboratory Practice (GLP) and the peer review publication process, and their relative contributions to ensuring information quality for regulatory decision-making. Some scientists who are engaged in the endocrine issue debate have expressed extreme disappointment that their research, which has not been conducted according to GLP standards, was unfairly given less weight by regulators. These scientists have been critical of GLP and have alleged that GLP is merely an expensive record keeping and study reporting requirement. The authors of this review conclude that the highly critical view of GLP is not supported by published analyses pointing to subjectivity and variability in peer-review processes. Although GLP is not designed to establish relative merit, it is an internationally accepted quality assurance, quality control method for documenting experimental conduct and data. The authors conclude that neither the peer review process nor GLP are completely sufficient for establishing relative scientific soundness. However, changes occurring both in peer-review processes and in regulatory guidance resulting in clearer, more transparent communication of scientific information point to an emerging convergence in ensuring information quality. The solution to determining relative merit lies in developing a well-documented, generally accepted WoE scheme to evaluate both peer-reviewed and GLP information used in regulatory decision making where both merit and specific relevance inform the process.Hypothesis-driven Weight of Evidence Framework for Evaluating Data within the U.S. EPA’s Endocrine Disruptor Screening Program.
C.J. Borgert, et al., Regul. Toxicol. Pharmacol. (2011) 61(2): 185-191.
The authors discuss why WoE approaches are preferred and often used to critically examine, prioritize, and integrate results from different types of studies to reach general conclusions. The authors conclude that for assessing hormonally active agents, WoE evaluations are necessary to assess screening assays that identify potential interactions with components of the endocrine system, long-term reproductive and developmental toxicity tests that define adverse effects, mode of action studies aimed at identifying toxicological pathways underlying adverse effects, and toxicity, exposure and pharmacokinetic data to characterize potential risks. The authors propose a hypothesis-driven WoE approach for hormonally active agents and illustrate the approach by constructing hypotheses for testing the premise that a substance interacts with various components of the endocrine system and for evaluating data within the U.S. Environmental Protection Agency’s (EPA) Endocrine Disruptor Screening Program (EDSP).Potency Matters: Thresholds Govern Endocrine Activity.
C.J. Borgert, et al., Regul. Toxicol. Pharmacol. (2013) 67(1): 83-88.
This article argues that thresholds exist for endocrine active substances and, therefore, safe levels of exposure to these substances can be established for protecting the health of humans and wildlife. Whether thresholds exist for endocrine active substances, and for endocrine disrupting effects of exogenous chemicals, has been posed as a question for regulatory policy by the EU. This question arises from a concern that the endocrine system is too complex to allow estimations of safe levels of exposure to any chemical with potential endocrine activity, and a belief that any such chemical can augment, retard, or disrupt the normal background activity of endogenous hormones. However, the authors conclude that vital signaling functions of the endocrine system require it to continuously discriminate the biological information conveyed by potent endogenous (i.e., internal to the body) hormones from a more concentrated background of structurally similar, exogenous (i.e., external to the body) molecules with low hormonal potential. This obligatory ability to discriminate important hormonal signals from background noise can be used to define thresholds for induction of hormonal effects, without which normal physiological functions would be impossible. From such thresholds, safe levels of exposure can be estimated. This brief review highlights how the fundamental principles governing hormonal effects – affinity, efficacy, potency, and mass action – dictate the existence of thresholds and why these principles also define the potential that exogenous chemicals might have to interfere with normal endocrine functioning.A Critique of the European Commission Document, “State of the Art Assessment of Endocrine Disruptors.”
L.R. Rhomberg, et al., Crit. Rev. Toxicol. (2012) 42(6): 465–473.
The authors critique a document titled "State of the Art Assessment of Endocrine Disrupters" (SOA Assessment) that was commissioned by the EU Directorate-General for the Environment to provide a basis for developing scientific criteria for identifying endocrine disruptors and reviewing and possibly revising the European Community Strategy on Endocrine Disrupters. The authors note that the SOA Assessment takes an anecdotal approach rather than attempting a comprehensive assessment of the state of the art or synthesis of current knowledge. To have accomplished the latter, the document would have had to (i) distinguish between apparent associations of outcomes with exposure and the inference of an endocrine-disruption (ED) basis for those outcomes; (ii) constitute a complete and unbiased survey of new literature since 2002 (when the WHO/IPCS document, "Global Assessment of the State-of-the-Science of Endocrine Disruptors" was published); (iii) consider strengths and weaknesses and issues in interpretation of the cited literature; (iv) follow a WoE methodology to evaluate evidence of ED; (v) document the evidence for its conclusions or the reasoning behind them; and (vi) present the evidence for or reasoning behind why conclusions that differ from those drawn in the 2002 WHO/IPCS document need to be changed. The authors conclude that, in its present form, the SOA Assessment fails to provide a balanced and critical assessment or synthesis of literature relevant to ED.Principles of Pharmacology and Toxicology Also Govern Effects of Chemicals on the Endocrine System
H. Autrup;, et al., Toxicol. Sci. (2015) 146(1): 11-15.
Authored by 15 distinguished academic scientists from the EU and the U.S., this article addresses a contention made by some that current paradigms used in toxicology and in hazard identification and risk characterization of chemicals are inadequate to protect people and wildlife from endocrine-disrupting chemicals (EDCs). The authors argue that chemicals with hormonal activity can be assessed by the well-evaluated health risk characterization approach used by governments and industry for many years.Critical comments on the WHO-UNEP State of the Science of Endocrine Disrupting Chemicals
2012. J.C. Lamb, et al., Regul. Toxicol. Pharmacol. (2014) 69(1): 22–40.
This article, written by U.S., Canadian and EU experts, provides a critique of a 2012 World Health Organization (WHO) report which purports to be an update to the highly regarded 2002 WHO State of the Science of Endocrine Disrupting Chemicals. First, the authors conclude the new WHO report is not a state-of-the-science review and does not follow the 2002 WHO recommended a Weight of Evidence (WoE) approach. Second, the authors note the report often presumes that endocrine disruption occurs based on exposure or a potential mechanism despite a lack of evidence to show that chemicals are causally established as endocrine disruptors. Additionally, causation is inferred by the presentation of a series of unrelated facts, which collectively do not demonstrate causation. Third, trends in disease incidence or prevalence are discussed without regard to known causes or risk factors; endocrine disruption is implicated as the reason for such trends in the absence of evidence. Fourth, dose and potency are ignored for most chemicals discussed. Finally, controversial topics (i.e., low-dose effects, non-monotonic dose response) are presented in a one-sided manner and these topics are important to understanding endocrine disruption. Overall, the authors conclude the 2012 report provides less than a balanced perspective, and does not accurately reflect the state of the science on endocrine disruption.